Aqueous zinc–iodine (Zn–I₂) batteries are regarded as highly promising energy storage systems due to their high safety, abundant resources, and low cost. Benefiting from the variable oxidation states of iodine, this system holds potential for high-capacity energy storage. However, conventional Zn–I₂ batteries primarily rely on the two-electron conversion between I⁻ and I⁰, which results in limited capacity, sluggish reaction kinetics, and pronounced shuttle effects. In recent years, strategies such as introducing halide ions (e.g., Cl⁻, Br⁻) to modulate iodine valence have enabled the formation of interhalogen compounds and multi electron reactions. Nevertheless, multi electron systems still face challenges such as hydrolysis of high valent iodine species, interfacial instability, and poor reversibility. Therefore, constructing systems that can stabilize high valent iodine intermediates and accelerate electrochemical reaction kinetics is a key pathway toward achieving highly efficient and reversible multi electron Zn–I₂ batteries.

Recently, a team led by Peiyi Wu and Yucong Jiao from the College of Chemistry and Chemical Engineering at Donghua University proposed a dual-electrode synergistic electrolyte (DESA-E), constructing a highly reversible multi-electron Zn–I₂ battery (Scheme 1).
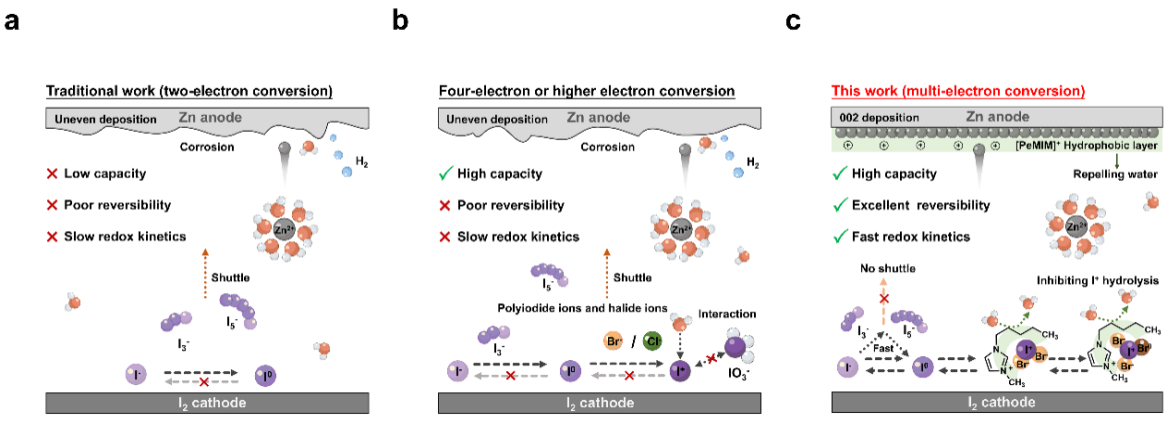
Scheme 1. Schematic diagram of different electron transfer reactions in Zn–I₂ batteries
The research team introduced [PeMIM]⁺Br⁻ into 2MZnSO₄ (baseline electrolyte, BE) to achieve synergistic regulation among halogens. This approach promoted the multi-electron conversion of I⁻/I⁰/I⁺ while significantly enhancing the utilization of the Br⁻/Br⁰ redox reaction (from 8.9% to 31.2%). The [PeMIM]⁺ cation creates a hydrophobic microenvironment that stabilizes high-valent iodine species. Meanwhile, its hydrophobic alkyl chain modulates zinc deposition, enabling highly reversible reactions at both the cathode and anode.

Figure 1 screening of imidazole bromides with different alkyl chain lengths
To identify the optimal imidazolium salt additive, the research team systematically compared a series of compounds from [EtMIM]⁺Br⁻ to [HeMIM]⁺Br⁻ with varying alkyl chain lengths. Theoretical calculations revealed that the [PeMIM]⁺Br⁻ system exhibits the highest hydrolysis energy barrier for I⁺ species and the strongest binding affinity toward IBr₂⁻, demonstrating superior stability. Consequently, 0.5M[PeMIM]⁺Br⁻ was determined as the optimal system (denoted as DESA-E) (Figure 1).

Figure 2 electrochemical mechanism of a dual halogen battery
The three consecutive reversible redox peaks observed under different voltage windows reveal the multi-electron conversion process, which proceeds from I⁻/I⁰ to I⁰/I⁺ and further involves Br⁻/Br⁰. The synergistic dual-halogen interaction significantly enhances the utilization of the Br⁻/Br⁰ redox couple (from 8.9% to 31.2%), as confirmed by XPS, in situ Raman spectroscopy, and theoretical calculations (Figure 2).

Figure 3 cathode redox kinetics under different electrolyte systems
Combined electrochemical and in situ spectroscopic results indicate that the DESA-E system significantly enhances the redox kinetics and multi-electron reaction reversibility of iodine, suppresses I₃⁻ accumulation, and reduces interfacial polarization, thereby enabling a rapid and stable multi-electron transfer process (Figure 3).
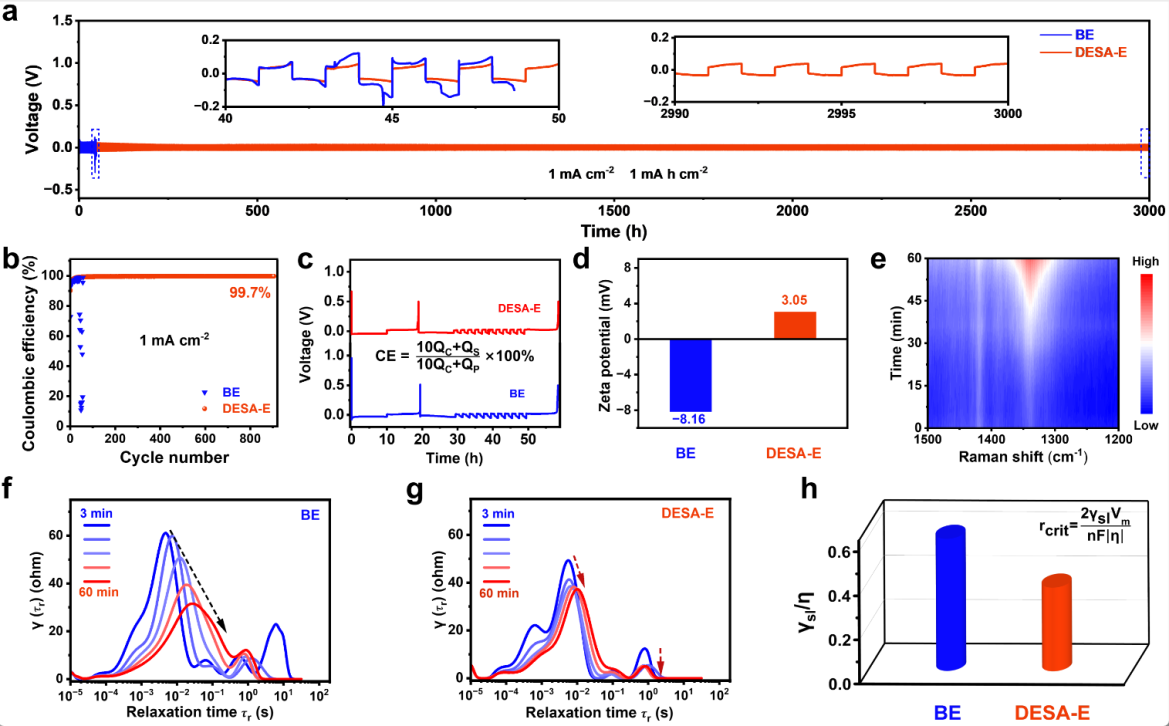
Figure 4 effect of BE and DESA-E on the electrochemical performance of Zn/Zn and Zn/Cu batteries
The DESA-E system significantly improves zinc deposition&stripping performance. The symmetric cell demonstrates stable cycling for 3,000 hours at 1 mA cm⁻², and the asymmetric cell achieves over 900 cycles, both far surpassing the conventional BE system. The adsorbed [PeMIM]⁺ on the zinc surface reduces water molecule adsorption and creates an electrostatic shielding effect, guiding uniform Zn²⁺ deposition and synergistically enhancing interfacial stability and reaction kinetics (Figure 4).
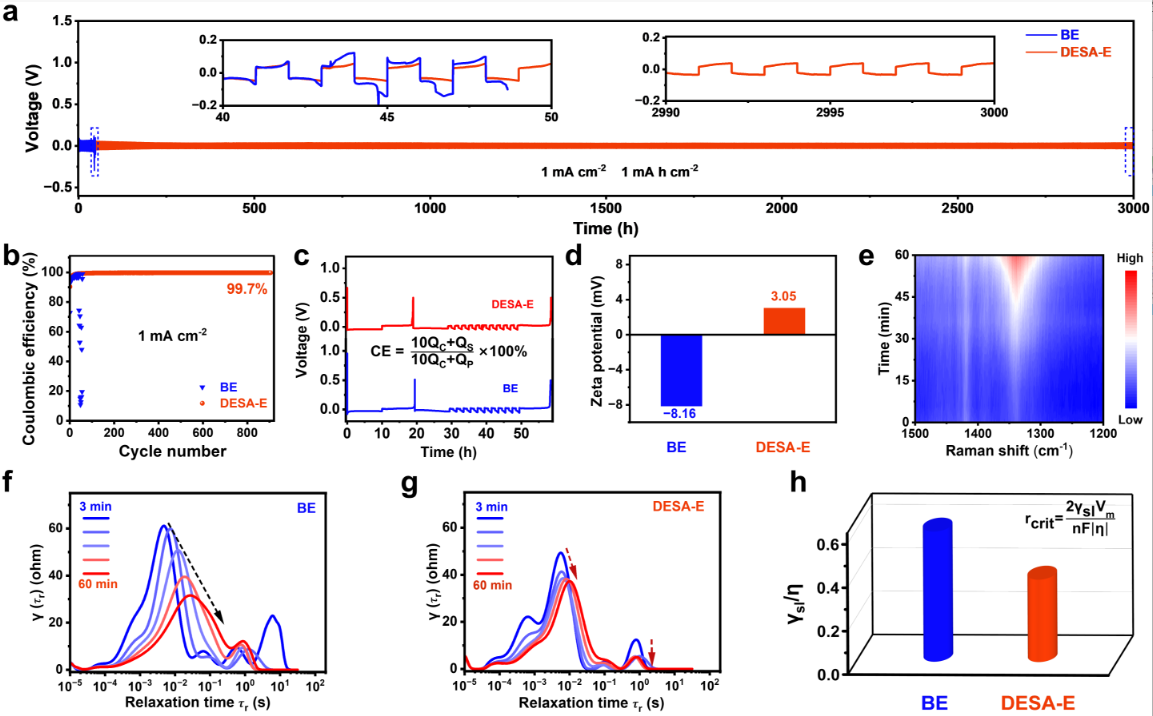
Figure 5 full-cell performance evaluation
Compared with previously reported Zn–I₂ battery systems, the DESA-E system significantly suppresses polyiodide shuttling and enables rapid multi electron conversion, demonstrating superior rate performance, cycling stability, and Coulombic efficiency. Its stable operation under high temperature and pouch cell conditions further highlights the practical potential of this multi electron transfer system (Figure 5).
Chen Xuan, a Ph.D. candidate at the College of Chemistry and Chemical Engineering, Donghua University, is the first author of the paper, with Ph.D. candidate Feng Doudou serving as a co first author. The corresponding authors are Researcher Jiao Yucong and Professor Wu Peiyi. The related research, entitled “Dual Electrode Synergistic Electrolyte Enabling Highly Reversible Multi Electron Redox in Aqueous Zinc–Iodine Batteries,” has recently been published in Advanced Materials (Adv. Mater.). This work was supported by the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, and the Natural Science Foundation of Shanghai, among other funding sources.
Original link to the article:https://doi.org/10.1002/adma.202513389
