
Academician Zhu Meifang, the project's strategic advisor and Director of the State Key Laboratory of Advanced Fiber Materials at Donghua University, explained that this research introduces the concept of ‘active’ fibrous neural probes and has created an implantable fiber platform that mimics the structure of an earthworm. This platform is highly compatible with biological tissues in its mechanical properties, being both stretchable and movable. It has successfully enabled controllable navigation of neural interfaces within the brain and muscular systems, allowing for dynamic, real-time, and long-term monitoring of neural electrical signals and biomechanical signals (demonstrated over 13 months within muscle). According to Researcher Yan Wei from the State Key Laboratory for Advanced Fiber Materials and the College of Materials Science and Engineering at Donghua University, this breakthrough marks a paradigm shift in neuroelectronics—from static implants to dynamic interaction, and from rigid devices to active, intelligent fiber systems. It paves a new path for future brain-computer interfaces, wearable diagnostic/therapeutic systems, and chronic disease neuromodulation.
Breakthrough: From ‘Fixed Probe’ to ‘Roaming Monitor’, Rewriting the Rules of Neural Interfaces
In nature, earthworms achieve highly distributed sensory and motor control capabilities through their unique segmented structure (metamerism). Each body segment contains discrete sensory and neural units that precisely detect external stimuli and respond flexibly, demonstrating remarkable adaptability for navigating complex environments. Drawing inspiration from this natural design, Researcher Liu Zhiyuan and Researcher Yan Wei's team at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, have developed NeuroWorm—a dynamic, soft, and stretchable fiber neural interface modeled after worm-like structures.
Traditional neural interfaces are characterized by their static nature. A key example is the electrode for Parkinson's disease therapy, which, after implantation, remains fixed in a single brain region. Monitoring other areas necessitates repeated invasive procedures for new electrode placement. The research team initially encountered comparable obstacles: their implantable metallic glass fibers with a feature size of 40 nm could accommodate no more than four sensors and suffered from limited flexibility, leading to significant biocompatibility issues and immune rejection as the body treated them as foreign bodies. The ‘neural worm’ represents a fundamental breakthrough against these limitations.
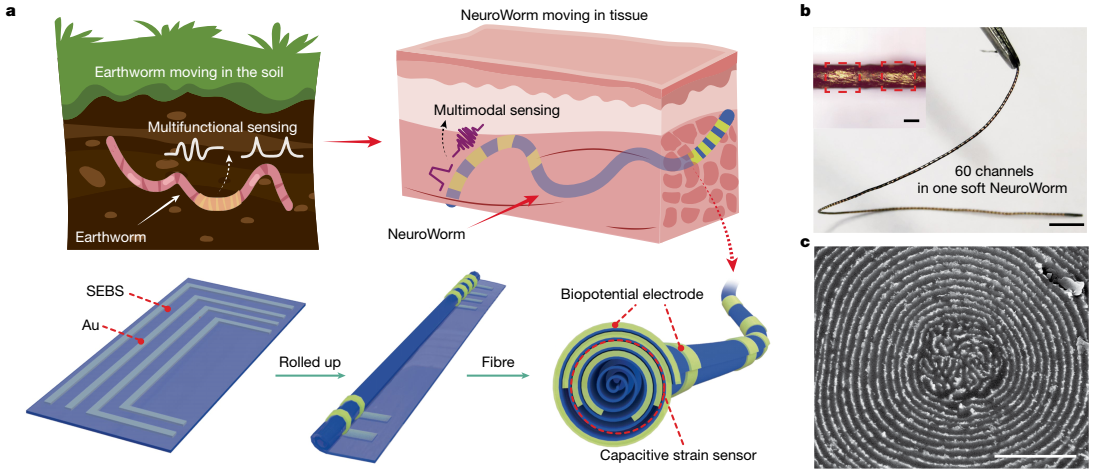
design, manufacturing strategy, and demonstration of ‘NeuroWorm’
A). Increased Sensor Density: The team fabricated a single, ~200-micrometer-diameter fiber integrating 60 axially distributed discrete electrodes and strain sensors, representing a 15-fold increase over conventional methods. This high-density, minimally invasive platform enables navigation within tissue and precise multi-point monitoring of neuroelectrical and biomechanical signals.
B). Enhanced Mechanical Compatibility: Fibers are emerging as a key platform in neural interfaces due to their softness, sub-hair-scale dimensions, and capacity for multi-material integration. The fiber substrate in this work exhibits mechanical properties highly matched to human tissue. It demonstrated excellent biocompatibility in animal models, residing in muscle for 13 months without encapsulation or visible trace upon extraction.
C). Incorporation of Locomotion: Researchers collaborated with the team of Researcher Xu Tiantian at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, to innovatively introduce an open-loop magnetic control strategy. This achieved preliminary controllable propulsion and steering of the NeuroWorm within tissue, allowing it to navigate obediently inside the body. In experimental videos, the transparent fiber, like an earthworm moving through soil, travels from one area to another in a lab mouse, leaving a clear trail of neural signal recordings—all without requiring a second surgery. This mobility is powered by the magic of magnetic control. "It's like a tiny robot roaming through the brain and muscles, entirely non-invasive and harmless to the body," explained Yan Wei, using gestures to illustrate.
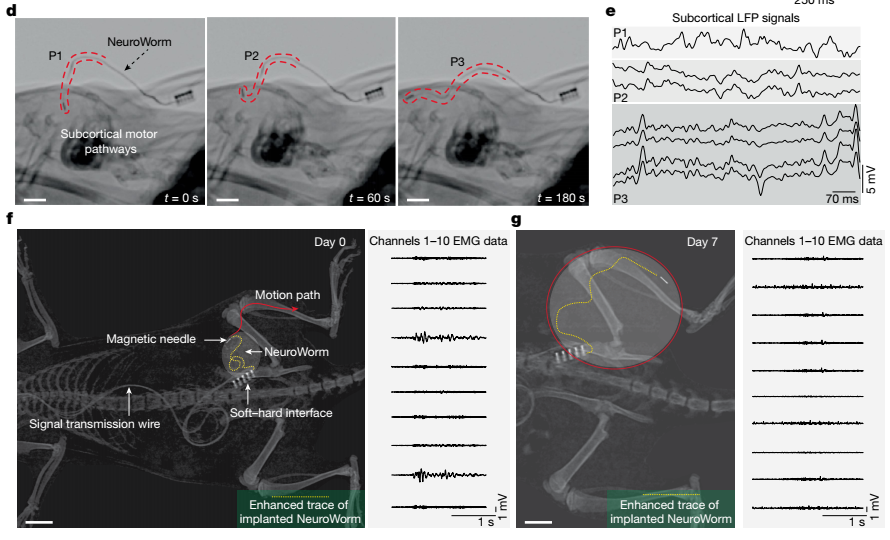
dynamic monitoring of the brain and subcutaneous fascia by NeuroWorm under magnetic field control
The Future: Sparing Parkinson's Patients from Repeat Surgeries, Offering Early Warnings for Neurological Diseases
The groundbreaking advancement of the fibrous ‘NeuroWorm’ is redefining the paradigm for treating neurological disorders. Traditionally, managing Parkinson's disease might require implanting multiple electrodes in different brain regions through separate surgeries, each carrying significant risks. The ‘NeuroWorm’, however, requires only a single implantation. It can then navigate to various affected areas, monitor neuroelectrical signals, and even effectively alleviate symptoms through electrical stimulation. This capability heralds a future where precise regulation of neural activity becomes a practical reality.
Its deeper value lies in its ‘long-term monitoring’ capability. In animal experiments, the research team implanted NeuroWorm into rat muscles through a minimal incision, continuously recording stable physiological signals for over 43 weeks. Even after 54 weeks of implantation, it showed no significant tissue reaction or fibrotic encapsulation, demonstrating exceptional long-term stability and biocompatibility rarely seen in similar devices. Compared to traditional clinical fiber-optic cables, NeuroWorm minimally disturbs surrounding tissues while delivering superior signal quality, revealing immense potential for clinical translation. Its 13-month in-vivo retention capability enables it to function like a ‘neural monitor,’ continuously capturing early signals of diseases like Parkinson's and Alzheimer's. “Much like wearable devices monitor heart rate, it can detect neural abnormalities early and issue warnings before symptoms manifest,” explained Yan Wei. This approach paves new pathways for ‘early intervention’ in neurological disorders.
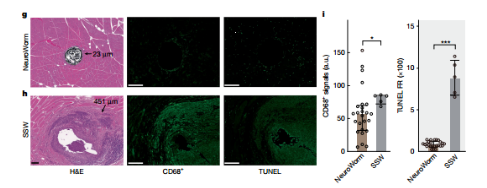
NeuroWorm Long-Term Biocompatibility Demonstration
Zhu Meifang revealed to reporters that the collaborative team will continue to deepen related research in the future. By constructing large electromagnetic coil arrays, they aim to create a high-intensity, dynamically adjustable magnetic field environment. Simultaneously, by integrating micro-magnetic needle arrays with closed-loop motion control strategies, the team seeks to achieve safe, precise, and real-time navigation and perception decoupling control within complex tissues. This breakthrough will propel bioelectronics from ‘fixed passive recording’ to a critical leap toward ‘mobile intelligent collaboration.’ The team anticipates collaborating closely with more application units in the future to accelerate the practical implementation of this technology.

It is reported that Yan Wei, a researcher at Donghua University, along with Liu Zhiyuan and Xu Tiantian, researchers at the Shenzhen Institute of Advanced Technology of the Chinese Academy of Sciences (SIAT), and Han Fei, an associate researcher at SIAT, jointly served as corresponding authors of the paper. The primary contributing institution was SIAT. Academicians Zhu Meifang and Zheng Hairong of the Chinese Academy of Sciences provided profound guidance and crucial support in key aspects including the project's overall design, refinement of research directions, and establishment of critical material systems. This research received funding and support from multiple sources, including the National Key R&D Program of China, the National Natural Science Foundation of China, the ShenzhenMunicipal Science and Technology Program, and the Chinese Academy of Sciences Strategic Priority Research Program.
