Recently, the research team led by Lingling Chu from the Center for Advanced Low-Dimensional Materials, in collaboration with the team of Bruce A. Arndtsen from McGill University in Canada, achieved significant progress in the field of enantioselective carbonylation coupling reactions. The findings were published in the Journal of the American Chemical Society. Ling Li, a doctoral student from our university, served as the first author of the paper, with Donghua University listed as the first corresponding institution.

Transition metal-catalyzed carbonyl coupling reactions play a pivotal role in drug discovery, natural product synthesis, and materials science. However, constructing the α-carbonyl chiral structures commonly found in drug molecules via asymmetric carbonyl coupling reactions between alkyl halides and nucleophiles remains challenging. This difficulty primarily stems from the strong coordination interaction between carbon monoxide and the transition metal nickel, which both impedes the effective activation of C(sp3)-halides and weakens the control of chiral ligands over the reaction's enantioselectivity.
This study innovatively proposes a strategy combining photo-oxoredox catalysis with chiral nickel catalysis, successfully achieving the first asymmetric carbonyl coupling reaction between benzyl and related C(sp³)-halides with amines. This enables the synthesis of a series of chiral amides with excellent enantioselectivity. By separately regulating the reactivity and stereoselectivity through photocatalysis and nickel catalysis, the research team effectively overcame the suppression of carbon monoxide on the oxidative addition of alkyl halides which is a challenge in conventional methods. Simultaneously, through meticulous optimization of chiral ligands and reaction conditions, the reaction achieved high efficiency and selectivity. The synthetic approach developed in this study can be applied to the preparation of pharmaceutical molecules such as nonsteroidal anti-inflammatory drugs, demonstrating significant value in both synthetic chemistry and drug discovery.
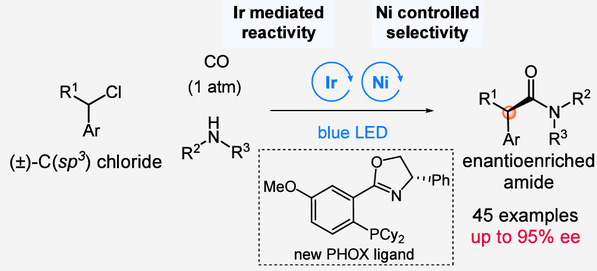
(Achieving Asymmetric Carbonyl Coupling Reactions via a Dual-Catalyst Strategy)
Original link to this article:https://pubs.acs.org/doi/10.1021/jacs.5c08527
