Rotator cuff injuries are a common musculoskeletal disorder, and traditional repair methods often face challenges such as high postoperative recurrence rates (13%–94%) and poor functional recovery. To address this clinical challenge, Professor Mo Xiumei's research team from the College of Biological Science and Medical Engineering at our university collaborated with the team led by Deputy Director Ji Yunhan from the Department of Orthopedics and Joint Surgery at Shanghai Tongren Hospital. Utilizing an innovative interdisciplinary project model, they conducted multidisciplinary collaborative research and developed a novel functional gradient-structured scaffold. This scaffold effectively mimics the natural tendon-bone interface, significantly enhancing the mechanical performance and therapeutic outcomes of rotator cuff repair.

Recently, research findings titled ‘Functionally graded scaffold with M2 macrophage-derived LncRNA-Encoded peptide: Mechanistic and therapeutic evaluation for rotator cuff repair’ were published online in the international materials science journal Bioactive Materials. The corresponding authors of the paper are Professor Mo Xiumei and Deputy Chief Physician Ji Yunhan. The co-first authors include Feng Hao, a graduate student from the College of Biological Science and Medical Engineering at Donghua University, and Zhang Gonghao and Xiong Li, attending physicians from Shanghai Tongren Hospital.
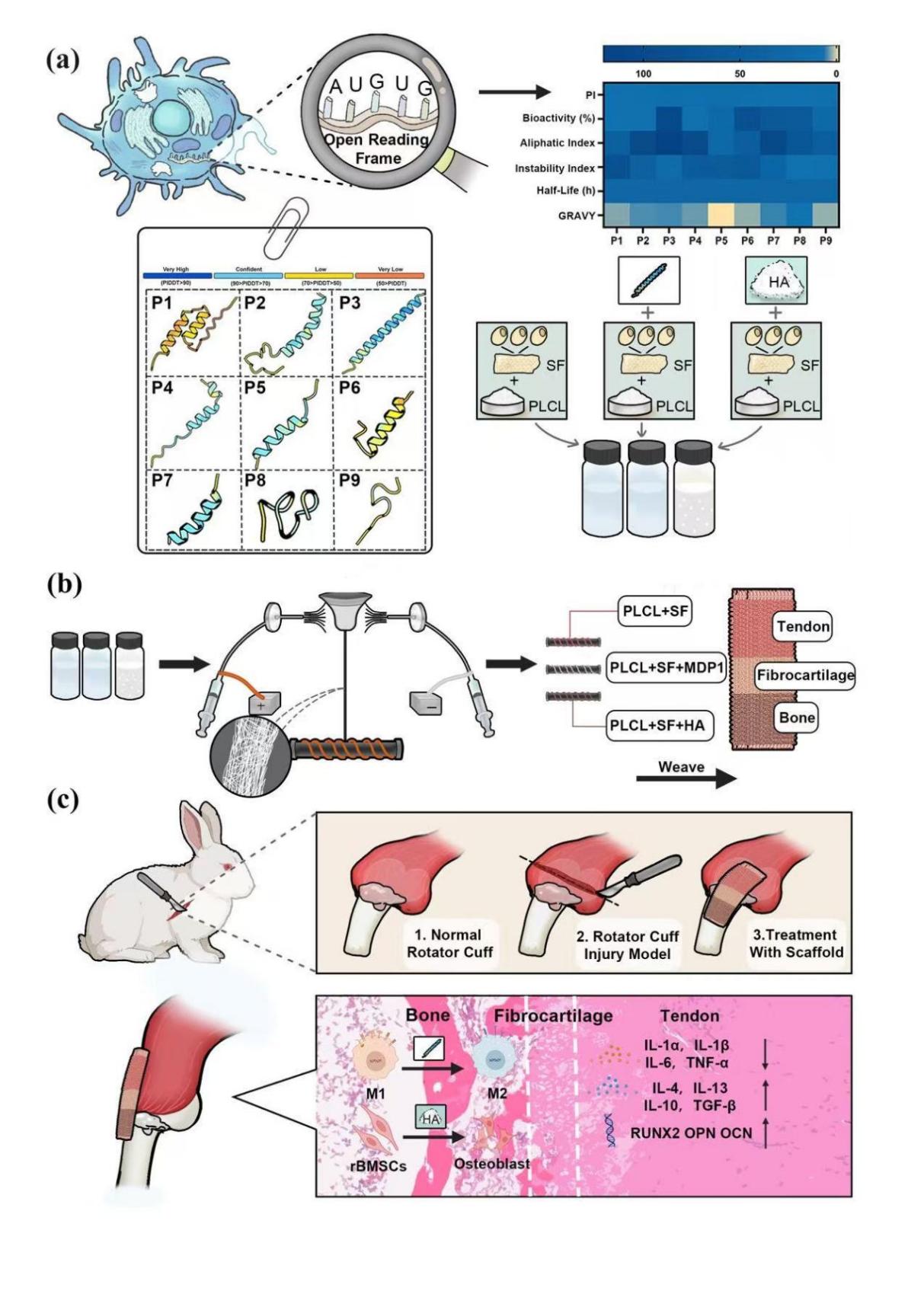
(Preparation of a functional graded scaffold for load MDP1 and its application in a rotator cuff injury model)
To address the clinical challenge of achieving secure healing of rotator cuff tendon tears to bone, the first functional gradient-structured scaffold has been successfully developed. This scaffold mimics the multi-layered structural characteristics of the human tendon-bone interface, achieving a repair effect closer to natural healing. The scaffold introduces MDP1, a peptide encoded by LncRNA derived from M2-type macrophages, which precisely regulates local inflammatory responses and effectively suppresses excessive inflammation. Experiments using a rabbit rotator cuff repair model showed that after scaffold implantation, the expression levels of the inflammatory factor IL-6 at the repair site decreased by approximately 60.6% and 66.5% at 2 and 4 months post-surgery respectively, significantly improving the local microenvironment and facilitating accelerated tissue healing. The scaffold also incorporates hydroxyapatite (HA) particles, enhancing biomineralization capacity and promoting tight integration between tendons and bones, facilitating faster and more robust wound healing. In terms of mechanical properties, the scaffold was fabricated using an electrospun nanofiber yarn process, achieving a Young's modulus of up to 280 MPa, which is close to the level of native tendons in New Zealand white rabbits. It combines excellent strength and flexibility while effectively reducing the risk of re-tearing during the early stages of repair. This innovative achievement combines structural biomimicry, precise anti-inflammatory properties, biomineralization, and superior mechanical characteristics to provide a new solution for the repair of tendon-bone interface injuries such as rotator cuff tears.
This study was supported by multiple research grants, including ‘Shanghai Tongren Hospital & Donghua University Medical-Engineering Interdisciplinary Project’ and the ‘China-Germany Science Foundation Research Exchange Center China-Germany Cooperation and Exchange Project,’ demonstrating the proactive role of universities and hospitals in promoting high-level regional collaborative innovation and fostering the integrated development of basic research and technology transfer. In the future, this functionally graded structural scaffold is expected to be applied in various tendon-bone interface repair surgeries, such as rotator cuff tear repair, Achilles tendon insertion point injuries, and cruciate ligament reconstruction, helping patients achieve more robust and physiologically natural healing outcomes. Further plans include conducting studies on larger animal models and preclinical research to optimize the scaffold preparation process and expand its indications, thereby accelerating the translation and application of this innovative achievement to benefit more patients in sports medicine and orthopedics.
Original link to the article:https://doi.org/10.1016/j.bioactmat.2025.06.032
