Sodium-ion batteries (SIBs) have become a promising next-generation secondary battery due to their abundant sodium resources, wide distribution, and low cost, and have great potential to complement lithium-ion batteries and ensure energy storage safety in the field of energy storage. However, compared with lithium ions, sodium ions have a larger ionic radius and atomic mass, and their mass-to-charge ratio (~23) is about 3.3 times that of lithium ions (~6.94). These properties result in a lower sodium ion transport rate. The non-uniform sodium ion flux results in low Coulombic efficiency (CE) and dendrite growth, which seriously affects the cycle life of SIBs. Therefore, improving the sodium ion transport kinetics is crucial for the practical application of SIBs. The current strategy to enhance ion transport near the anode is mainly through modulating the solvent-borne structures to reduce the desolvation energy when ions migrate from the liquid phase to the solid phase, which can effectively enhance the cation transfer kinetics and thus alleviate some of the inherent problems of the battery. However, according to the principle of charge conservation and the law of electroneutrality, the total current in the electrolyte solution is equal to the sum of the charges carried by the cations and anions migrating under the action of the electric field, and thus the traditional method of modulating the solvation structure to enhance the kinetics is getting closer and closer to the theoretical limit.
Aiming at the critical problem that the unstable anode interface in sodium batteries drastically shortens the cycle life of the batteries, a scientific research team from the National Key Laboratory of Advanced Fiber Materials and the School of Materials Science and Engineering proposed a method of high dipole moment diaphragm to modulate the interface components of the anode solid electrolyte, which has been published in AngewandteChemie International Edition, 2025, 64, e202415283. Recently, the team proposed a strategy to optimize the solvation structure based on the diaphragm material. By developing anion-anchored diaphragms, free anions in the bulk phase of the cell are effectively anchored and the migration of anions in the system is reduced, thus improving the diffusion flux of sodium ions. The results of ion mobility kinetics confirm that the developed anion-anchored diaphragm effectively reduces the anions migrating to the positive electrode, and is able to effectively weaken the solvation effect of anions and increase the proportion of solvent-separated ion pairs (SSIPs) in the solvated structure. This work was published as ‘Enhancing Robustness and ChargeTransfer Kinetics of Sodium-ion Batteries through Introduction of AnionicAnchoring Separators’ in the Journal of the American Chemical Society, 2025, 147, 8488-8499. The paper was correspondingly authored by Meifang Zhu and Guiyin Xu, with the first author being M.S. student Xiaoxiao Li, and Ph.D. student Tao Zhang as a co-author.
During the discharge process, free sodium ions and SSIPs have a positive charge and migrate toward the positive pole under the influence of the electric field, promoting current transfer. Meanwhile, some of the aggregates containing anions (AGG) have negative charges and migrate toward the negative electrode, thus contributing negatively to the current transfer (Fig. 1a). This suggests that anions reduce the contribution of Na+ to electrode current transfer and increase the internal resistance of the battery. To address these issues, an anion-anchored septum (AAS) was elaborated to modulate the solvation structure of the bulk phase in the cell, and the solvation structure of Na+ was analyzed by solvated cluster stoichiometric probability density. The results demonstrate that the proposed anion-anchoring strategy effectively confines anions and promotes the formation of SSIPs, which can serve as the main charge carriers, thus potentially improving the charge transfer kinetics in SIBs.
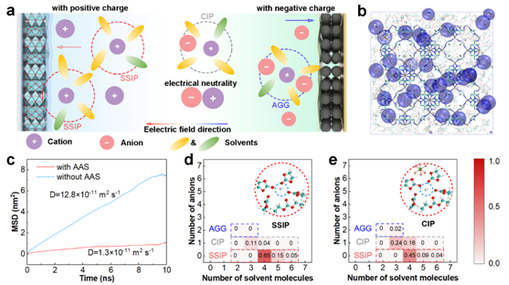
Figure 1: Simulation of the effect of anionic anchoring and solvated structures.
The team developed a Hitov method incorporating nuclear magnetic resonance (NMR) to analyze the ion transport process under the action of an electric field and quantified the anion migration kinetics on a time scale (Fig. 2d). The results confirmed that AAS can effectively inhibit PF6- transport. Although this method has the limitation of unrealistic volume ratio of diaphragm to electrolyte. But it also suggests that the anchoring effect of AAS on anions may be better than the test results. Meanwhile, the effect of AAS on ion transport kinetics was further verified using in situ attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR).
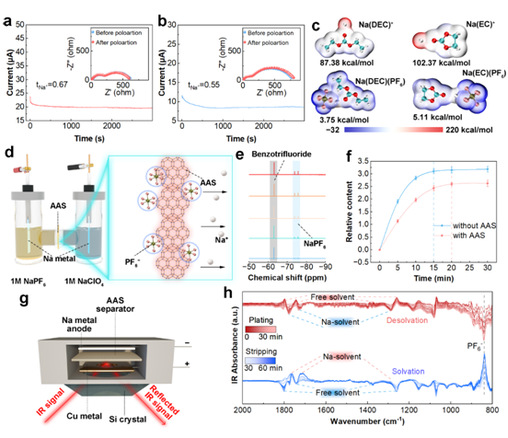
Figure 2: Study of the effect of AAS on Na+ transport kinetics.
The excellent Na+ transport kinetics induced by AAS is expected to improve the negative electrode stability and reversibility of the battery. The sand’s time of the Na||Cu cell with AAS was longer (3.55 h vs. 1.25 h for GF) (Fig. 3a). In addition, more dense and uniform Na deposition morphology was observed after 50 cycles in Na||Na symmetric cells (Fig. 3b). Na+ plating/stripping tests were performed in Na||Na symmetric cells (Figs. 3d, 3e), and the cells assembled with AAS exhibited good reversibility and lower overpotential. The anion-anchoring strategy reduces the internal resistance and improves the reversibility of plating/stripping, which results in higher critical current density (CCD) and coulombic efficiency (CE).The AAS makes plating stripping remarkably reversible, and thus it operates well even under the harsh conditions with a very low positive-to-negative capacity ratio. These results show that the anion-anchoring strategy improves the stability of the negative electrode, effectively reduces the cell volume and increases the energy density.
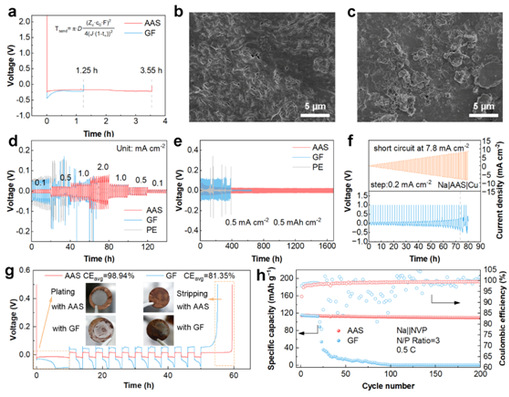
Figure 3: Characterization of negative electrode stability and plating/stripping reversibility.
The anion decreases the oxidative stability of the solvent, which affects the stability of the CEI. Linear scanning voltammetry (LSV) showed that anionic domain limitation led to an increase in the oxidation potential, which effectively mitigated the parasitic reaction at the positive electrode of the cell (Fig. 4c). A thinner and more homogeneous CEI was also formed when equipped with AAS. at low E/C ratios, the Na|AAS|NVP cells exhibited superior cycling stability, which can be attributed to the fact that AAS reduces the risk of negative electrode failure and mitigates the side reactions in the electrolyte (Fig. 4f). To further elucidate the mechanism of AAS-induced generation of stable CEI, X-ray photoelectron spectroscopy (XPS) tests were performed on the negative electrode (Figs. 4i, 4j). The results showed that the CEI film formed in the GF system had a high organic and low inorganic content compared with the AAS system, which implied that the CEI layer formed in the GF was thicker and more resistant, indicating that this strategy could effectively improve the oxidative stability of the solvent to generate uniform and stable CEI.
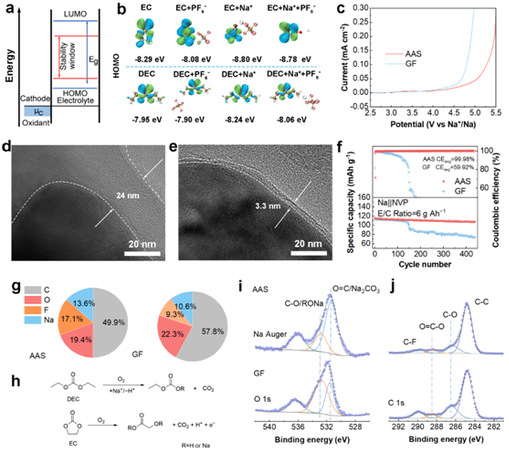
Figure 4: Effect of AAS on positive electrode stability.
At 0.5 C, the HC|AAS|NVP full cell exhibited less capacity degradation during cycling, achieving a capacity retention of 91.35% and an average Coulombic efficiency of 99.5% (Figure 5c). Meanwhile, the assembled HC|AAS|NVP soft-packed cell, with an energy density of up to 225.7 Wh kg-1 at 0.2 C (based on the mass of the whole cell), exhibited high capacity (176 mAh) and excellent cycling stability (Fig. 5f). In summary, AAS enables sodium-ion batteries to ensure cycling stability while using a lean electrolyte with a high positive electrode loading. This reduces the volume and mass of the inactive components in the battery, which reduces the cost, increases the energy density, and provides a new technological solution for long-cycle, high-energy sodium-ion batteries.
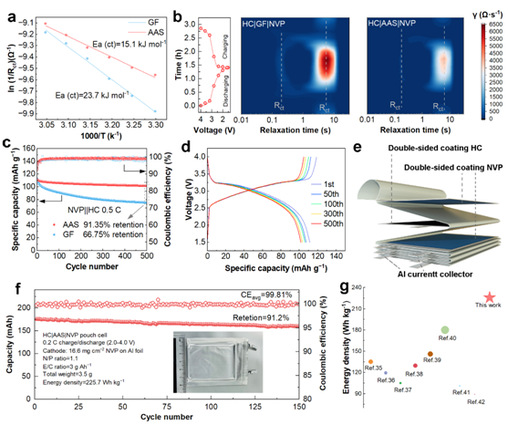
Figure 5: Performance of the full HC|AAS|NVP cell.
In summary, An anion confinement strategy was successfully developed. The proposed anion-anchored diaphragm effectively anchors free anions, increases the proportion of SSIP in the solvent, mitigates the side reactions of the positive electrode, and reduces the cation transport energy barrier of the negative electrode. As a result, Na||Na symmetric batteries with AAS can be stabilized for Na plating/stripping for more than 1,600 hours under equal capacity and current density conditions of 0.5 mAhcm-2 and 0.5 mA cm-2. Stable cycling performance can also be achieved when equipped with AAS under electrolyte-poor and low N/P ratio conditions, resulting in a high energy density of up to 225.7 Wh kg-1 for HC|AAS|NVP soft-packed batteries.This strategy opens up a new avenue for the design of functional diaphragms for the realization of next-generation safe and rechargeable sodium-ion batteries. This work was funded by the National Key R&D Program of China (2022YFB3803502), the National Natural Science Foundation of China (52103076), the International Cooperation Fund of Shanghai Municipal Science and Technology Commission (24520713300), and the Shanghai Qi-Star Program (24QA2700100).
Link of full article: https://pubs.acs.org/doi/10.1021/jacs.4c16227
