Li Jingchao’s research team from the College of Biological Science and Medical Engineering has long been engaged in studying the biological effects of nanomaterials and precise, efficient cancer therapy. Focusing on the scientific challenge of improving the “efficacy” and “precision” of antitumor conjugated polymer materials through molecular structure regulation and multifunctionalization, the team has conducted in-depth research on the structure-performance relationship of conjugated polymers and proposed a series of innovative strategies to enhance their antitumor properties. Recently, the team has made multiple research advances in precise and efficient cancer treatment.
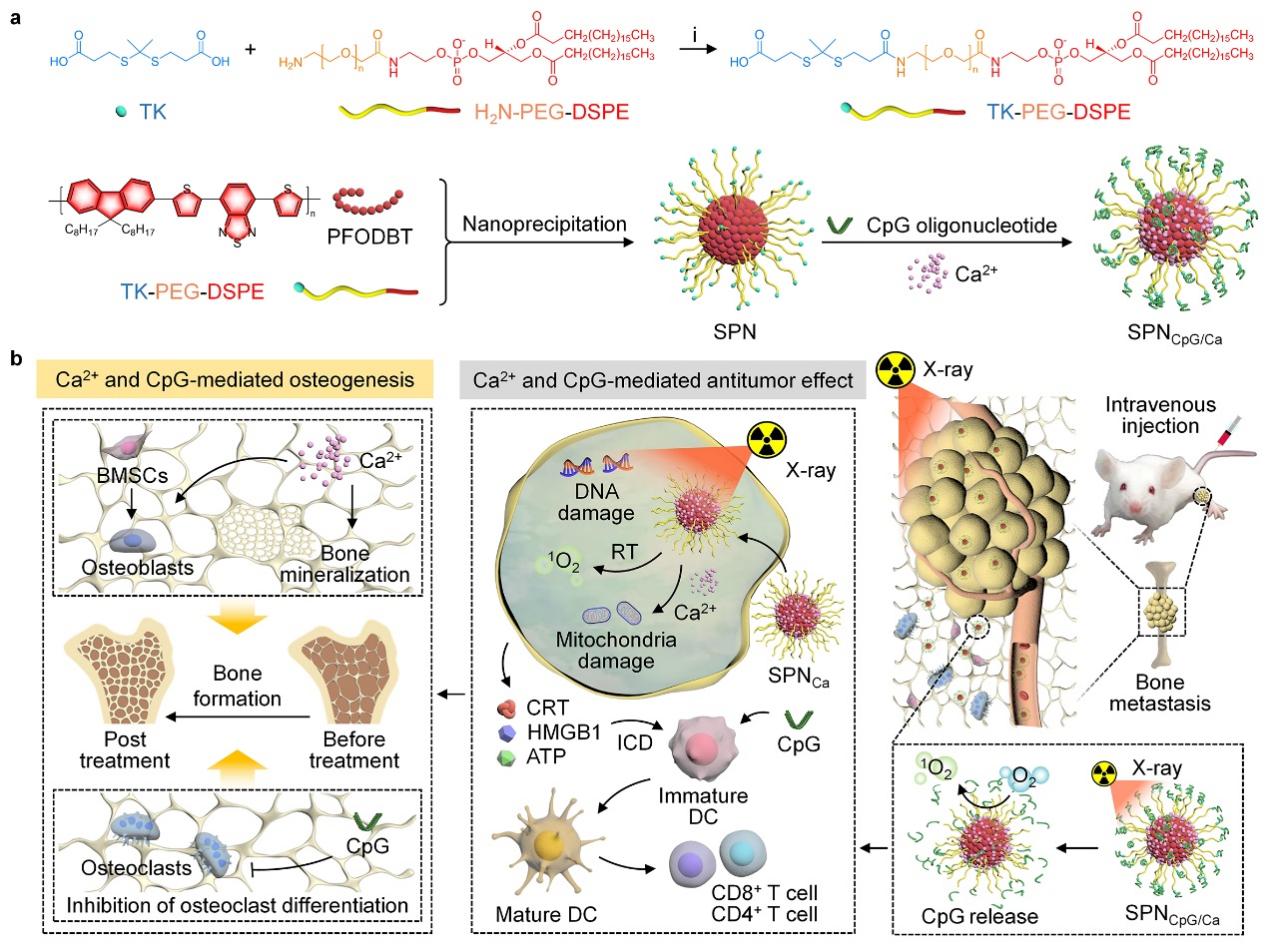
Addressing the clinical challenge of bone metastasis treatment, Li Jingchao’s team designed a multifunctional semiconductor nano-integrator (SPNCpG/Ca) that combines radiotherapy, calcium overload, and immunotherapy to treat bone metastases and repair tumor-associated bone damage. Semiconductor polymer nanoparticles (SPN) act as radiosensitizers producing reactive oxygen species (ROS) during radiotherapy, which also break ROS-responsive fragments to release the immunoadjuvant CpG. Thus, ROS generation, calcium overload, and CpG release mediated by SPNCpG/Ca induce cell death and immune activation. In a breast cancer bone metastasis mouse model, this combined treatment effectively inhibited bone metastasis growth and liver and lung metastases. Additionally, Ca2+ accelerates osteogenic differentiation of bone marrow mesenchymal stem cells, while CpG suppresses osteoclast differentiation in the bone metastatic microenvironment to alleviate bone resorption, synergistically promoting repair of tumor-associated bone damage. This multifunctional therapeutic nanosystem provides a new strategy for treating bone metastases and repairing tumor-related bone destruction. The research was published in Advanced Functional Materials under the title “Breast Cancer Bone Metastasis Therapy and Tumor-Associated Bone Destruction Repair by Versatile Semiconducting Nanointegrators with X-ray Adjuvant.”
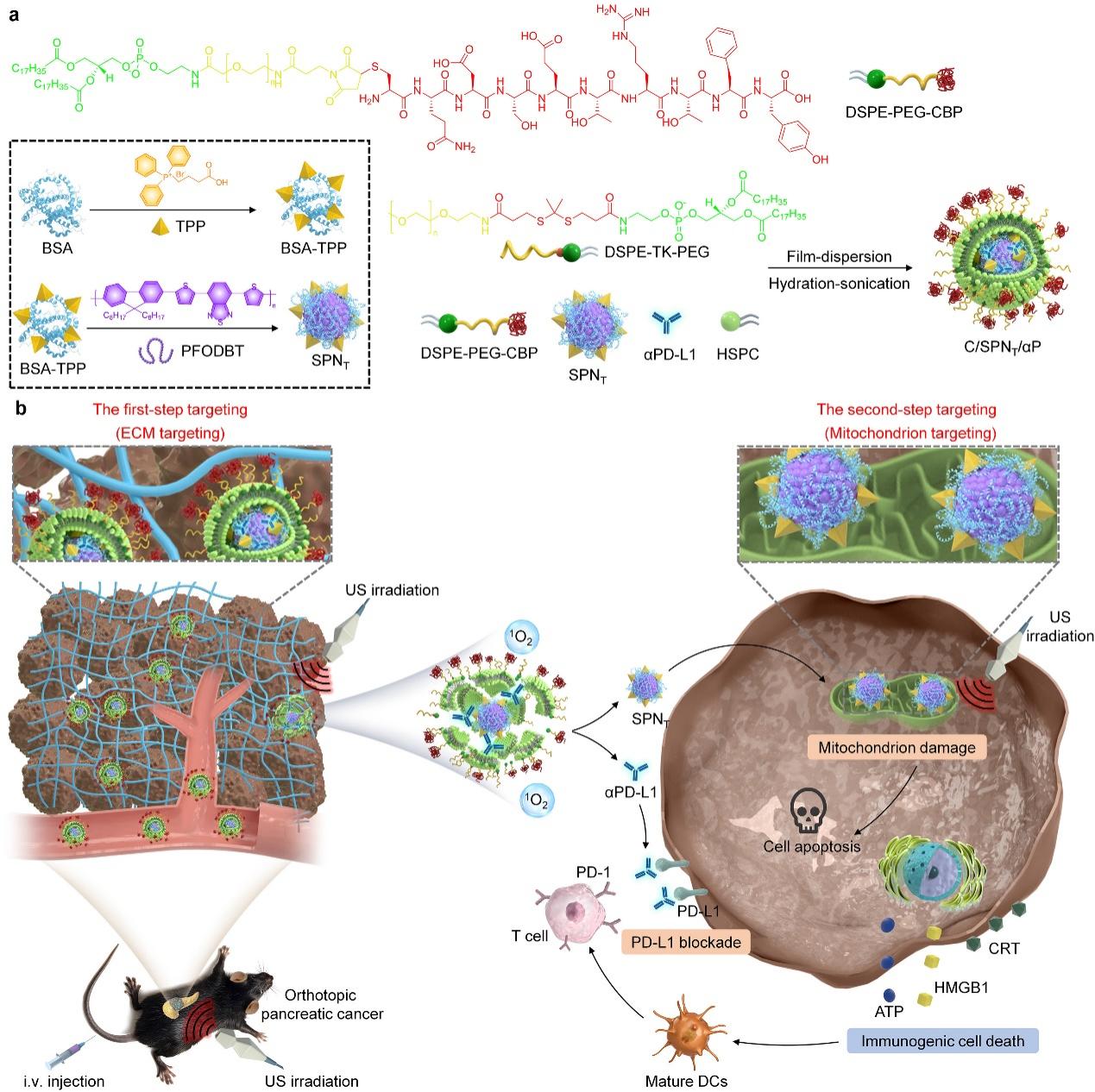
To address the clinical treatment challenges of pancreatic cancer, Li Jingchao’s team designed a semiconductor nano-transducer (C/SPNT/αP) that achieves orthotopic pancreatic cancer therapy by amplifying mitochondrial damage and programmed death ligand 1 (PD-L1) blockade. The team embedded small nanoparticles containing a mitochondria-targeting ligand (TPP) and semiconductor polymer (SP) (SPNT), along with αPD-L1, into ultrasound-responsive collagen-binding peptide (CBP)-conjugated nanoliposomes to form C/SPNT/αP. In the first step of extracellular matrix (ECM) targeting, CBP on the surface of C/SPNT/αP effectively binds collagen in the ECM, enhancing nanoparticle accumulation at the orthotopic pancreatic tumor site. Upon ultrasound (US) irradiation, C/SPNT/αP undergoes sonodynamic therapy (SDT), rupturing its ultrasound-responsive shell to release SPNT and αPD-L1 on demand. Subsequently, SPNT mediates mitochondrial targeting and amplifies mitochondrial damage under US irradiation by generating singlet oxygen (1O2), thereby enhancing apoptosis and immunogenic cell death (ICD) effects to promote immune activation. The immune effect of ICD is further enhanced by αPD-L1 release, which blocks the PD-L1 immunosuppressive pathway. This novel therapeutic strategy effectively treats orthotopic pancreatic tumors in Panc02 and KPC mouse models. The research was published in Advanced Functional Materials under the title Two-Step Targeting-Tunable Semiconducting Nanoswitches Amplify Mitochondrion Damage and PD-L1 Blockade for Orthotopic Pancreatic Cancer Therapy.
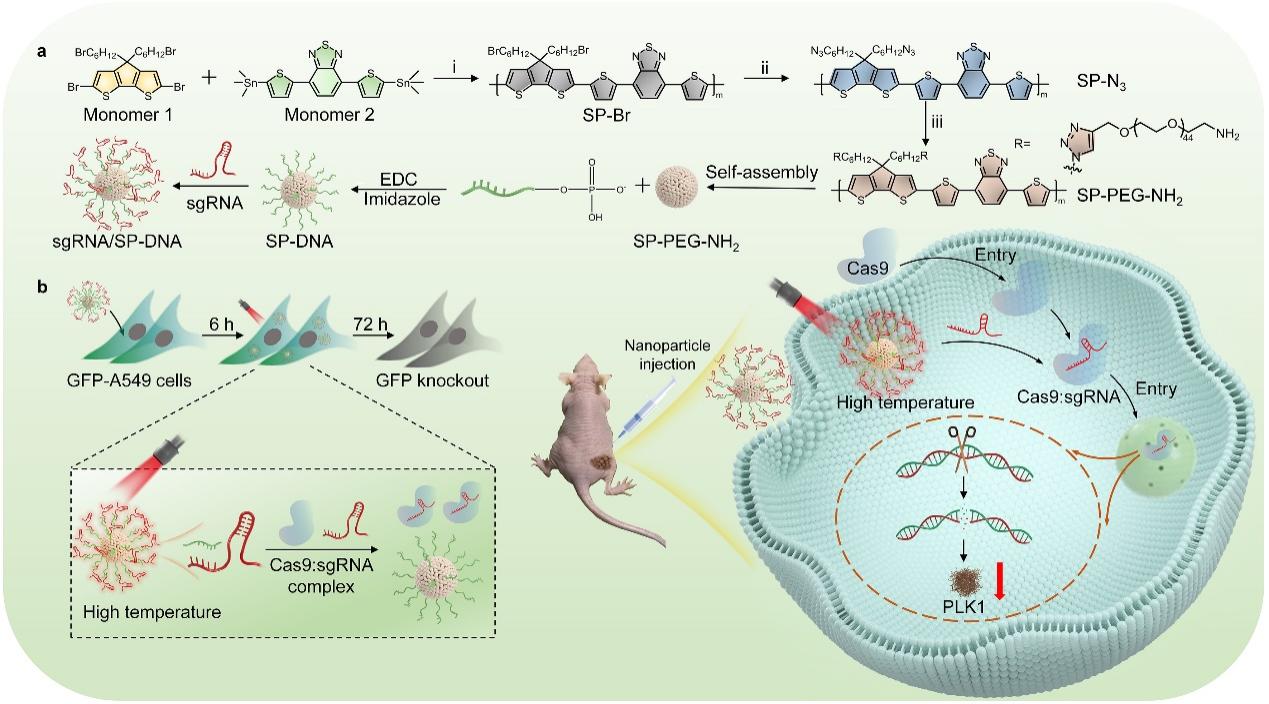
To address the critical clinical translation issue of achieving specific enrichment of CRISPR at lesion sites while avoiding off-target effects, Li Jingchao’s research team developed a near-infrared (NIR) light-responsive organic polymer nanocarrier based on semiconductor polymers for precise regulation of CRISPR activity and tumor gene therapy. This was achieved by grafting single-stranded DNA complementary to the sgRNA targeting region onto the side chains of semiconductor polymers (SP) with excellent photothermal conversion performance, forming a single-stranded DNA-modified carrier (SP-DNA). Based on complementary base pairing, the single-stranded DNA on SP-DNA hybridizes with sgRNA containing complementary base sequences to form an sgRNA/SP-DNA nanosystem. While fulfilling the delivery function, this nanocarrier acts as a NIR photothermal conversion medium, converting light energy into heat, causing a local temperature increase near the sgRNA/SP-DNA nanosystem. This thermal effect leads to the dissociation of sgRNA from the single-stranded DNA, releasing free sgRNA and thereby achieving precise control of CRISPR/Cas9 activity. This research was recently published in Nano Letters under the title A Semiconducting Polymer NanoCRISPR for Near-Infrared Photoactivatable Gene Editing and Cancer Gene Therapy.
